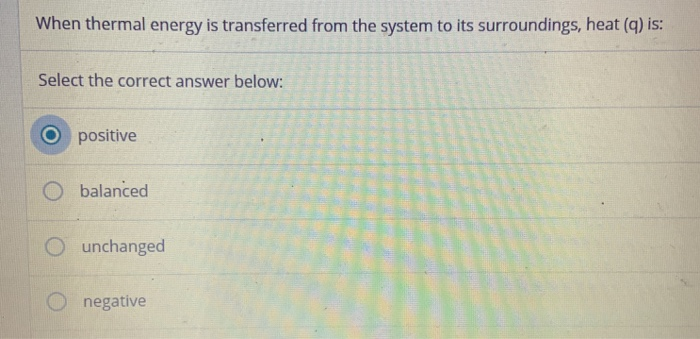
If Heat Is Transferred From The System To The Surroundings Then
If heat is transferred from the system to the surroundings then. In an exothermic process heat is transferred from the surroundings to the system. MCQ ON SECOND LAW OF THERMODYNAMICS. When a system_____ delta E is always negative.
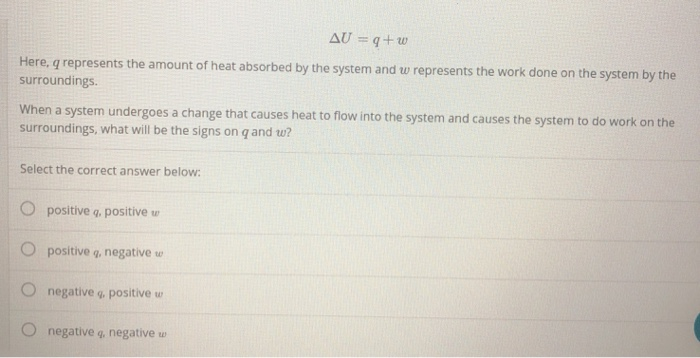
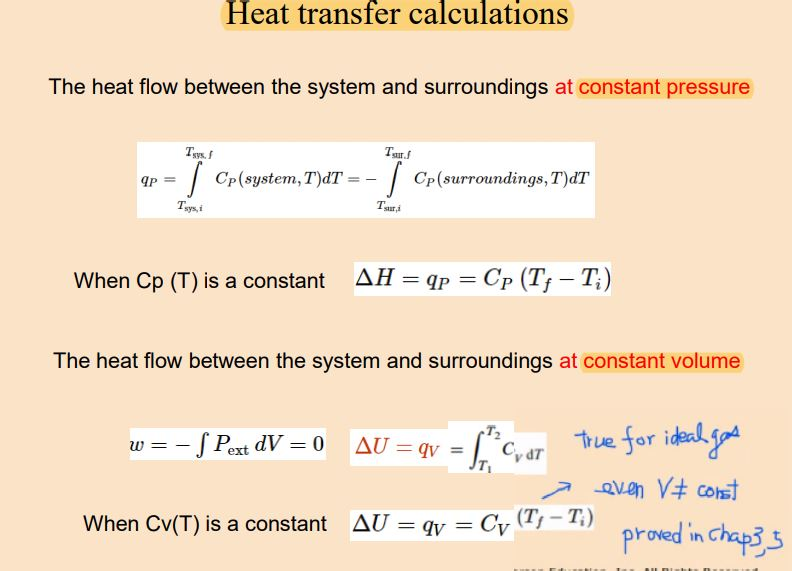
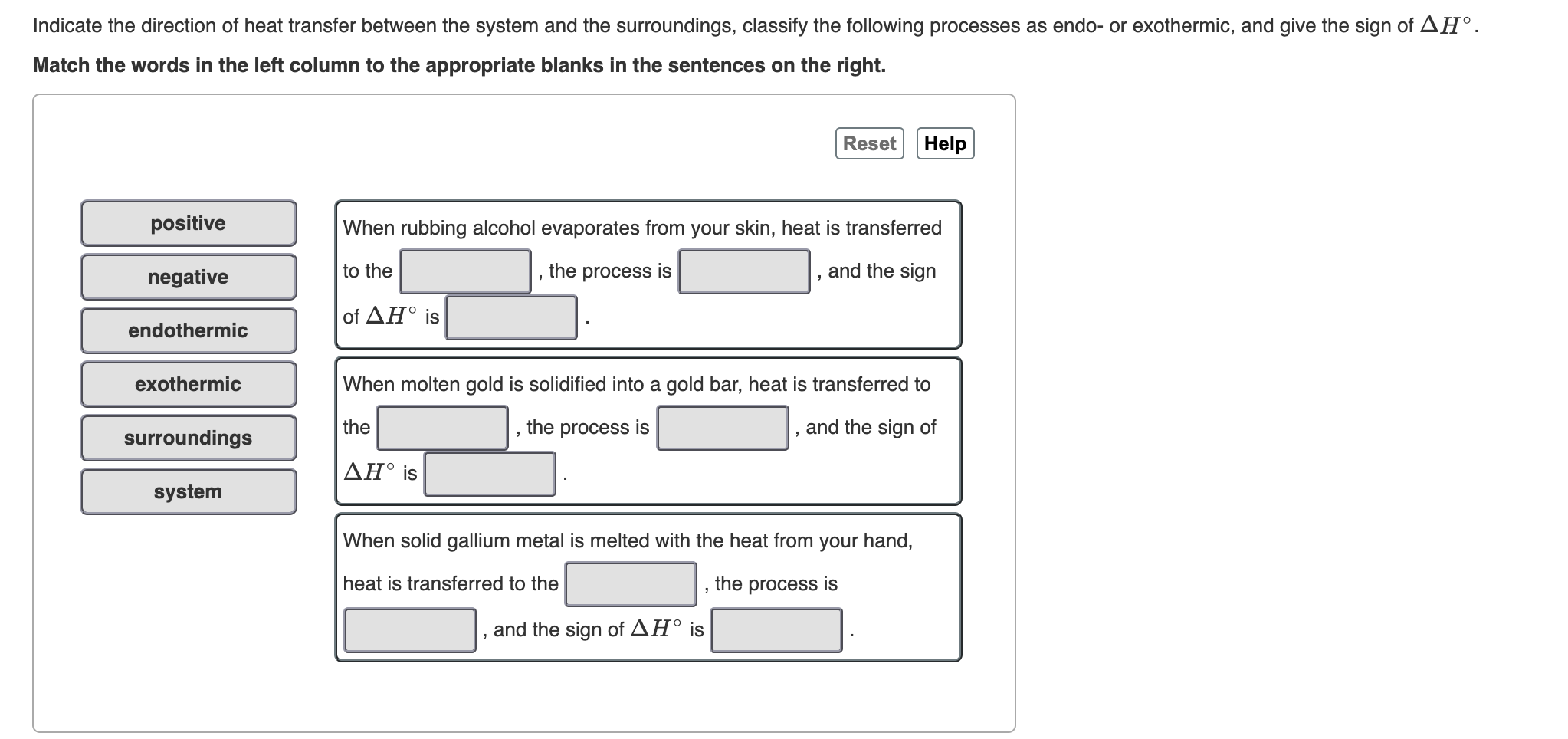
When energy is transferred as heat from the surroundings to the system ΔH is negative. If Wis positive then there is net work done by the system. Q system -q surroundings.
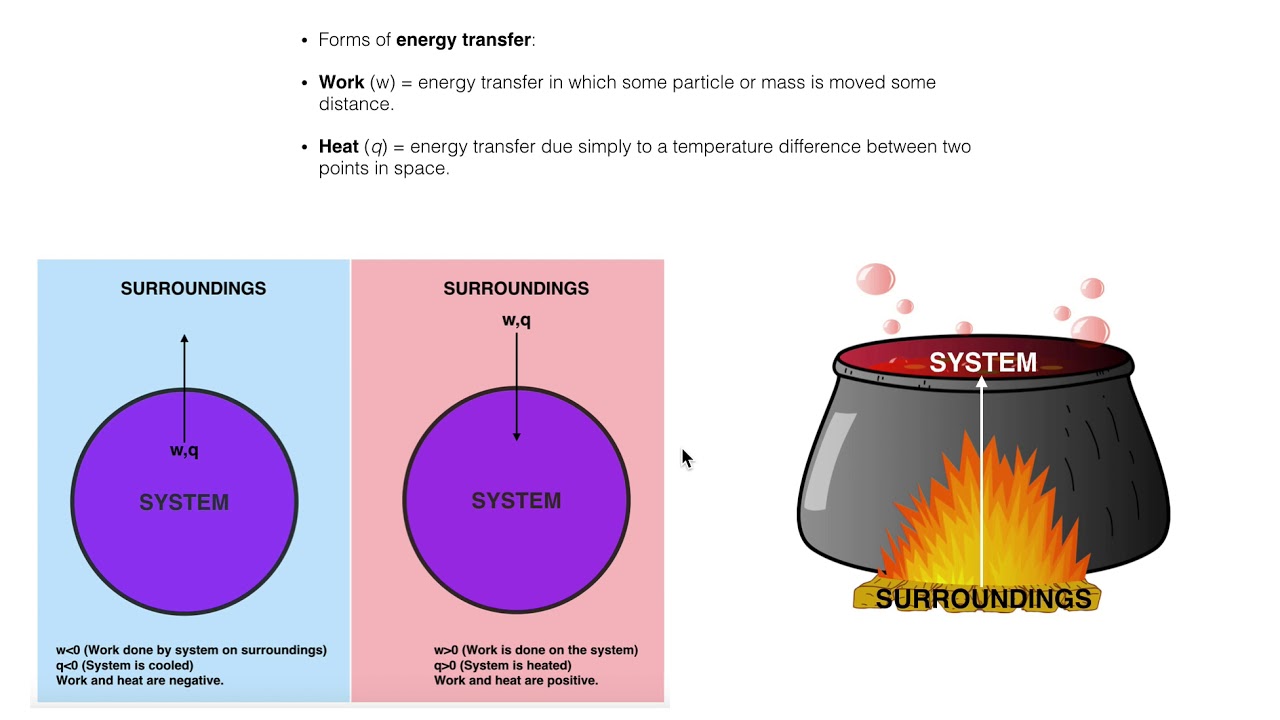
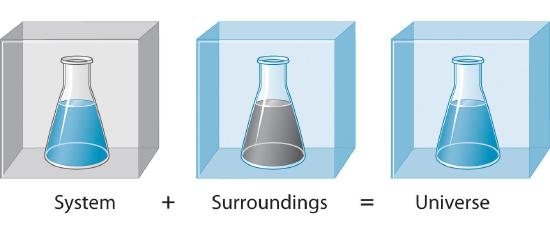
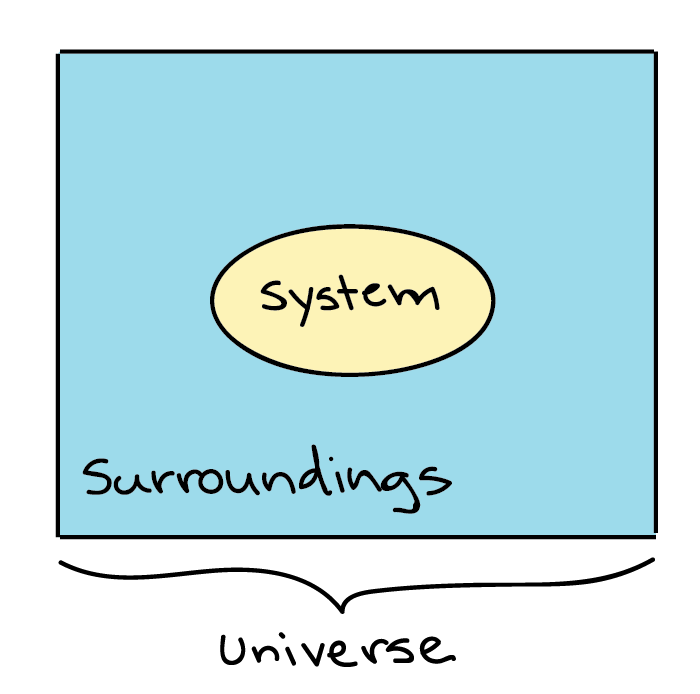
Heat transfer by convection occurs when a fluid such as air or water is in contact with an object whose temperature is higher than the temperature of its surroundings. Although there is heat transferred between the system and surroundings there is no heat transferred into or out of the universe and q universe is zero. - Heat and work are transfers of energy across a system boundary.
For example if any system undergoes a heat transfer process and all the time the temperature difference between system and surrounding is approximately zero it means that there is no entropy generation into the surroundingNow if we reverse the process then we will get an exact initial condition which we had at initially before heat transfer because there was no entropy generationIf we had entropy. The evaporation of water is an exothermic process. The total change in entropy system plus surroundings is therefore.
Heat is transferred to a heat engine from a furnace at a rate of 80 MW. If the rate of waste heat rejection to a nearby river is 50 MW determine the net power output for this heat engine. A absorbs heat and does work b gives off heat and does work c gives off heat and has work done on it d absorbs heat and has work done on it e none of the above.
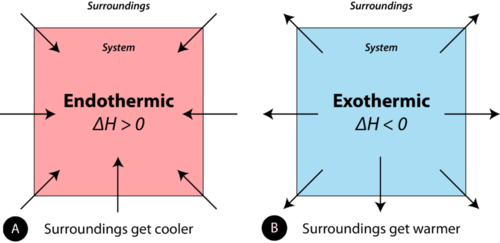
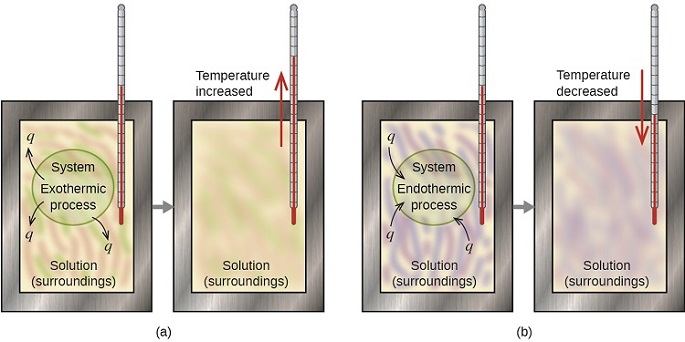
C energy 1 degree celsius. Qsystem is negative and qsurroundings is positive e. The surroundings will feel warmer in an endothermic process.
If ΔH is positive the process absorbs heat from the surroundings and is said to be endothermic. Uthe total energy contained within a system.
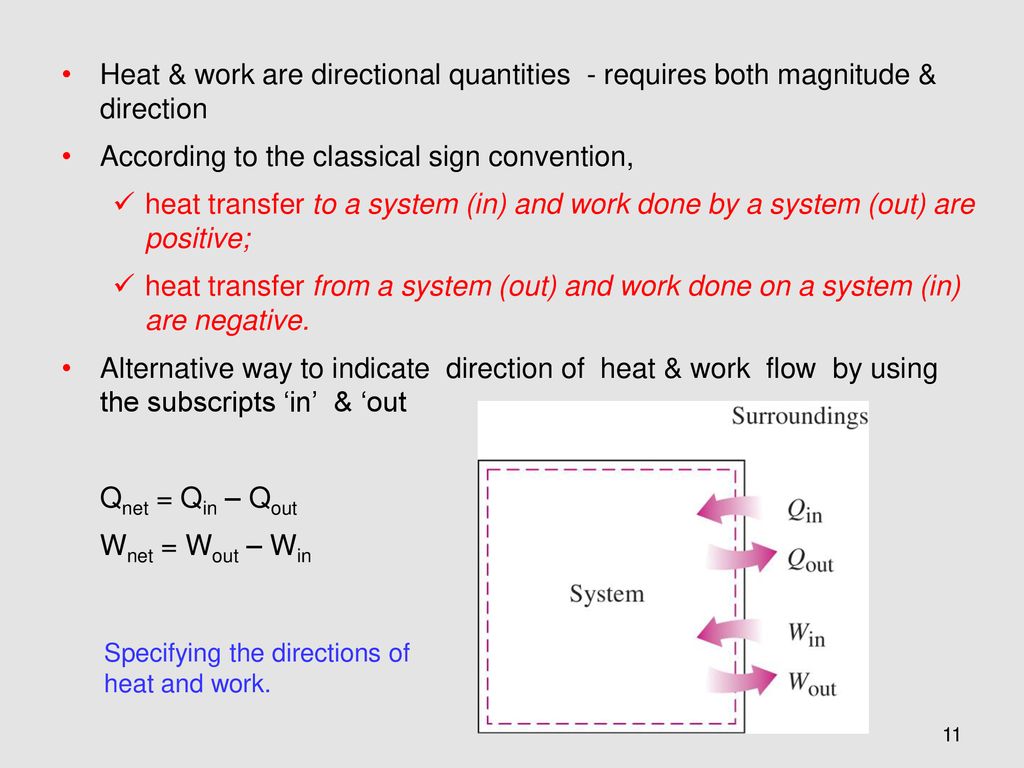
Q heat J WWork j if Q is positive then there is a net heat transfer into the system.
Is defined to be positive if it is transferred to the system. Heat transfer is a discipline of thermal engineering that concerns the generation use conversion and exchange of thermal energy between physical systems. Qsystem and qsurroundings are both positive. A combustion reaction is exothermic. Q heat J WWork j if Q is positive then there is a net heat transfer into the system. If Wis positive then there is net work done by the system. The surroundings will feel warmer in an endothermic process. When a system_____ delta E is always negative. ΔH for an endothermic reaction is positive.
Q heat J WWork j if Q is positive then there is a net heat transfer into the system. In an exothermic process heat is transferred from the surroundings to the system. So positive Q adds energy to the system and positive W. The surroundings will feel cooler in an exothermic process. ΔH for an endothermic reaction is positive. Consider the reverse reaction namely the formation of H2g and O2g from H2Ol. The evaporation of water is an exothermic process.


















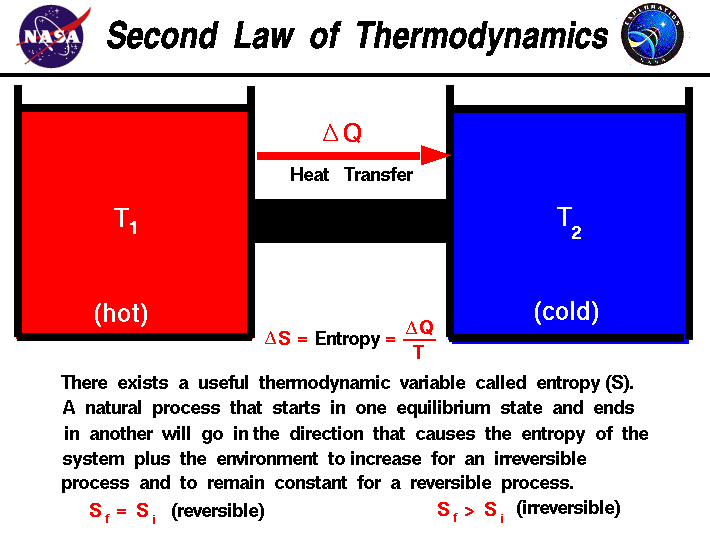









Post a Comment for "If Heat Is Transferred From The System To The Surroundings Then"